Abstract
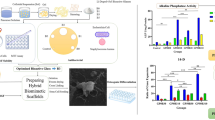
Modern techniques for expanding stem cells play a substantial role in tissue engineering: the raw material that facilitates regeneration of damaged tissues and treats diseases. The environmental conditions and bioprocessing methods are the primary determinants of the rate of cultured stem cell proliferation. Bioceramic scaffolds made of calcium phosphate are effective substrates for optimal cell proliferation. The present study investigates the effects of two bioceramic scaffolds on proliferating cells in culture media. One scaffold was made of hydroxyapatite and the other was a mixture of hydroxyapatite and ferromagnetic material (Fe3O4 nanoparticles). Disk-shaped (10 mm × 2 mm) samples of the two scaffolds were prepared. Primary human fibroblast proliferation was 1.8- and 2.5-fold faster, respectively, when cultured in the presence of hydroxyapatite or ferrous nanoparticle/hydroxyapatite mixtures. Optical microscopy images revealed that the increased proliferation was due to enhanced cell-cell contact. The presence of magnetic Fe3O4 nanoparticles in the ceramic scaffolds significantly increased cell proliferation compared to hydroxyapatite scaffolds and tissue culture polystyrene.










Similar content being viewed by others
References
A.J. Mothe and C.H. Tator, Advances in Stem Cell Therapy for Spinal Cord Injury, J. Clin. Invest., 2012, 122(11), p 3824–3834
F. Berthiaume, T.J. Maguire, and M.L. Yarmush, Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges, Ann. Rev. Chem. Biomol. Eng., 2011, 2, p 403–430
M. Mimeault and S.K. Batra, Concise Review: Recent Advances on the Significance of Stem Cells in Tissue Regeneration and Cancer Therapies, Stem Cells, 2006, 24, p 2319–2345
A.M. Parr, C.H. Tator, and A. Keating, Bone Marrow-Derived Mesenchymal Stromal Cells for the Repair of Central Nervous System Injury, Bone Marrow Transpl., 2007, 40, p 609–619
K.E. Hatzistergos, H. Quevedo, B.N. Oskouei, H. Qinghua, G.S. Feigenbaum, I.S. Margitich, R. Mazhari et al., Bone Marrow Mesenchymal Stem Cells Stimulate Cardiac Stem Cell Proliferation and Differentiation Novelty and Significance, Circ. Res., 2010, 107, p 913–922
M. Körbling and Z. Estrov, Adult Stem Cells for Tissue Repair—A New Therapeutic Concept, N. Engl. J. Med., 2003, 349, p 570–582
C.P. Hodgkinson, J.A. Gomez, M. Mirotsou, and V.J. Dzau, Genetic Engineering of Mesenchymal Stem Cells and its Application in Human Disease Therapy, Hum. Gene Ther., 2010, 21, p 1513–1526
S. Polgar, L. Karimi, and M.E. Morris, Stem Cell Therapy for Parkinson’ s disease: Are Double-Blind Randomized Control Trials the Best Design for Quantifying Therapy Outcomes?, J. Neurol. Neurophysiol., 2013, 4, p 170. doi:10.4172/2155-9562.1000170
I. García-Gómez, G. Elvira, A.G. Zapata, M.L. Lamana, M. Ramírez, J. García Castro, M. García Arranz, A. Vicente, J. Bueren, and D. García-Olmo, Mesenchymal Stem Cells: Biological Properties and Clinical Applications, Expert Opin. Biol. Ther., 2010, 10(10), p 1453–1468
L. Mazzini, K. Mareschi, I. Ferrero, E. Vassallo, G. Oliveri, N. Nasuelli, G.D. Oggioni, L. Testa, and F. Fagioli, Stem Cell Treatment in Amyotrophic Lateral Sclerosis, J. Neurol. Sci., 2008, 265, p 78–83
Y. Ikada, Challenges in Tissue Engineering, J. R. Soc. Interface, 2006, 3, p 589–601
A.D. Ebert and C.N. Svendsen, Human Stem Cells and Drug Screening: Opportunities and Challenges, Nat. Rev. Drug Discov., 2010, 9, p 367–372
Y. Ikada, Tissue Engineering: Fundamentals and Applications, Vol 46, Elsevier, San Diego, 2011
H. Patil, I.S. Chandel, A.K. Rastogi, and P. Srivastava, Studies on a Novel Bioreactor Design for Chondrocyte Culture, International Journal of Tissue Engineering, 2013, 2013, p 1–7
J.E. Hambor, Bioreactor Design and Bioprocess Controls for Industrialized Cell Processing, BioProcess Int., 2012, 10, p 22–33
M. Serra, C. Brito, C. Correia, and P.M. Alves, Process Engineering of Human Pluripotent Stem Cells for Clinical Application, Trends Biotechnol., 2012, 30, p 350–359
A.C. Allori, A.M. Sailon, and S.M. Warren, Biological Basis of Bone Formation, Remodeling, and Repair—Part I: Biochemical Signaling Molecules, Tissue Eng. B, 2008, 14, p 259–273
G.M. Harbers and D.W. Grainger, Cell-Material Interactions: Fundamental Design Issues for Tissue Engineering and Clinical Considerations. An Introduction to Biomaterials, Taylor Francis Group, Boca Raton, FL, 2006, p 15–45
G. Huang, L. Wang, S.Q. Wang, Y. Han, W. Jinhui, Q. Zhang, X. Feng, and T.J. Lu, Engineering Three-Dimensional Cell Mechanical Microenvironment with Hydrogels, Biofabrication, 2012, 4, p 042001
S. Mashayekhan, M. Hajiabbas, and A. Fallah, Stem Cells in Tissue Engineering, 2013, doi:10.5772/54371
T. Mygind, M. Stiehler, A. Baatrup, H. Li, X. Zou, A. Flyvbjerg et al., Mesenchymal Stem Cell Ingrowth and Differentiation on Coralline Hydroxyapatite Scaffolds, Biomaterials, 2007, 28(6), p 1036–1047
D. Turhani, E. Watzinger, M. Weissenbock, B. Cvikl, D. Thurnher, G. Wittwer et al., Analysis of Cell-Seeded 3-Dimensional Bone Constructs Manufactured In Vitro with Hydroxyapatite Granules Obtained from Red Algae, J. Oral Maxillofac. Surg., 2005, 63, p 673–681
F.H. Liu, Fabrication of Bioceramic Bone Scaffolds for Tissue Engineering, J. Mater. Eng. Perform., 2014, 23, p 3762–3769
J.G. Ocampo, M.E. Jaramillo, D.E. Sierra, and C.O. Orozco, Suspension Rheology, Porosity and Mechanical Strength of Porous Hydroxyapatite Obtained by Gel-casting and Infiltration, J. Mater. Eng. Perform., 2016, 25, p 431–442
F. Bistolfi, Radiazioni non ionizzanti, ordine, disordine e biostrutture, Minerva Medica, Torino, 1989, p 209–246
E. Neumann, Membrane Electroporation: Toward a Molecular Mechanism. Electricity and Magnetism in Biology and Medicine, University of Bielefeld, Bielefeld, 1992
Y. Mouneimne, Electroinsertion of Proteins into Membranes: A Novel Approach to the Study of Membrane Receptors, Harvard University, USA. Electricity and Magnetism in Biology and Medicine, University of Bielefeld, San Francisco, 1992
C.E. Lindgren, Capturing the Aura Integrating Science, Technology and Metaphysics, Motilal Banarsidass Publishe, New Delhi, 2008
N. Dekhtyar, N. Polyaka, R. Sammons, 14th Baltic Conference on Biomedical Engineering and Medical Physics, Vol. 20, Springer, Berlin, 2008
D. Kumar, J.P. Gittings, I.G. Turner, C.R. Bowen, A. . Bastida-Hidalgo, and S.H. Cartmell, Polarization of Hydroxyapatite: Influence on Osteoblast Cell Proliferation, Acta Biomater., 2010, 6, p 1549–1554
S. Bodhak, S. Bose, and A. Bandyopadhyay, Bone Cell-Material Interactions on Metal-Ion Doped Polarized Hydroxyapatite, Mater. Sci. Eng., C, 2011, 31, p 755–761
W.R. Adey, Electromagnetics in Biology and Medicine, Modern Radio Science, H. Matsumoto, Ed., Oxford University Press, Oxford, 1993, p 245–277
T.Y. Tsong, Deciphering the Language of Cells, Trends Biochem. Sci., 1989, 14, p 89–92
Z.J. Sienkiewicz, N.A. Cridland, C.I. Kowalczuk, and R.D. Saunders, Biological Effects of Electromagnetic Fields and Radiation, The Review of Radio Science 1990–1992, M.R. Stone, Ed., Oxford Science Publications, Oxford, 1993, p 737–770
A. Kodama, N. Kamei, G. Kamei, W. Kongcharoensombat, S. Ohkawa, A. Nakabayashi, and M. Ochi, In Vivo Bioluminescence Imaging of Transplanted Bone Marrow Mesenchymal Stromal Cells Using a Magnetic Delivery System in a Rat Fracture Model, Br. J. Bone Joint Surg., 2012, 94, p 998–1006
J.I. Jacobson, R. Gorman, W.S. Yamanashi, B.B. Saxena, and L. Clayton, Low-Amplitude, Extremely Low Frequency Magnetic Fields for the Treatment of Osteoarthritic Knees: A Double-Blind Clinical Study, Altern. Ther. Health Med., 2001, 7(5), p 54–64
R. Zboril, M. Mashlan, and D. Petridis, Iron(III) Oxides from Thermal Processes Synthesis, Structural and Magnetic Properties, Mössbauer Spectroscopy Characterization, and Applications, Chem. Mater., 2002, 14(3), p 969–982
H.M. Kothari, E.A. Kulp, S.J. Limmer, P. Poizot, E.W. Bohannan, and J.A. Switzer, Electrochemical Deposition and Characterization of Fe3O4 Films Produced by the Reduction of Fe(III)-Triethanolamine, J. Mater. Res., 2006, 21(1), p 293–301
M.E. Bahrololoom, M. Javidi, S. Javadpour, and J. Ma, Characterisation of Natural Hydroxyapatite Extracted from Bovine Cortical Bone Ash, J. Ceram. Process. Res., 2009, 10, p 129–138
K. Haberko, M.M. Bucko, J. Brzezińska-Miecznik, M. Haberko, W. Mozgawa, T. Panz, A. Pyda, and J. Zar, ebski, Natural Hydroxyapatite—Its Behaviour During Heat Treatment, J. Eur. Ceram. Soc., 2006, 26, p 537–542
Y. Li, C.T. Nam, and C.P. Ooi, Iron (III) and Manganese (II) Substituted Hydroxyapatite Nanoparticles: Characterization and Cytotoxicity Analysis, J. Phys., 2009, 187(1), p 012024
R.P. Franke and F. Jung, Interaction of Blood Components and Blood Cells with Body Foreign, Surfaces, Ser. Biomech., 2012, 27, p 51–58
Genel Histoloji Erkocak, Dag Okan, Yay ltd. sti, Istanbul, 1983
R. Glicklis, L. Shapiro, R. Agbaria, J.C. Merchuk, and S. Cohen, Hepatocyte Behavior Within Three-Dimensional Porous Alginate Scaffolds, Biotechnol. Bioeng., 2000, 67, p 344–353
E. McCafferty, Relationship Between the Isoelectric Point (pHpzc) and the Potential of Zero Charge (E pzc) for Passive Metals, Electrochim. Acta, 2010, 55, p 1630–1637
M. Kosmulski, pH-Dependent Surface Charging and Points of Zero Charge, J. Colloid Interface Sci., 2006, 298, p 730–741
M. Kosmulski, The pH-Dependent Surface Charging and Points of Zero Charge, J. Colloid Interface Sci., 2011, 353, p 1–15
F. Bistolfi, Campi magnetici in medicina, Ed. Minerva Medica, Torino, 1993
C.W. Smith and S. Best, Electromagnetic Man, J.M. Dent & Sonns, London, 1989
F. Bistolfi, , Radiazioni non ionizzanti, ordine, disordine e biostruture, Ed. Minerva Medica, Torino, 1989
M. Cifra, J.Z. Fields, and A. Farhadi, Electromagnetic Cellular Interactions, Prog. Biophys. Mol. Biol., 2011, 105, p 223–246
H. Fröhlich, The Extraordinary Dielectric Properties of Biological Materials and the Action of Enzymes, Proc. Natl. Acad. Sci., 1975, 72, p 4211–4215
C. Rossi, A. Foletti, A. Magnani, and S. Lamponi, New Perspectives in Cell Communication: Bioelectromagnetic Interactions, Semin. Cancer Biol., 2011, 21, p 207–214
S. Seckiner Gorgun, Studies on the Interaction Between Electromagnetic Fields and Living Matter Neoplastic Cellular Culture, Front. Prospect., 1998, 7(2), p 1–21
L.-Y. Sun, D.-K. Hsieh, Y. Tzai-Chiu, H.-T. Chiu, L. Sheng-Fen, G.-H. Luo, T.K. Kuo, O.K. Lee, and T.W. Chiou, Effect of Pulsed Electromagnetic Field on the Proliferation and Differentiation Potential of Human Bone Marrow Mesenchymal Stem Cells, Bioelectromagnetics, 2009, 30, p 251–260
M.A. Omar, Elementary Solid State Physics, Pearson Education India, New Delhi, 1999
W. Adey, Biological Effects of Electromagnetic Fields, J. Cell. Biochem., 1993, 51, p 410–416
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maleki-Ghaleh, H., Aghaie, E., Nadernezhad, A. et al. Influence of Fe3O4 Nanoparticles in Hydroxyapatite Scaffolds on Proliferation of Primary Human Fibroblast Cells. J. of Materi Eng and Perform 25, 2331–2339 (2016). https://doi.org/10.1007/s11665-016-2086-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-016-2086-4